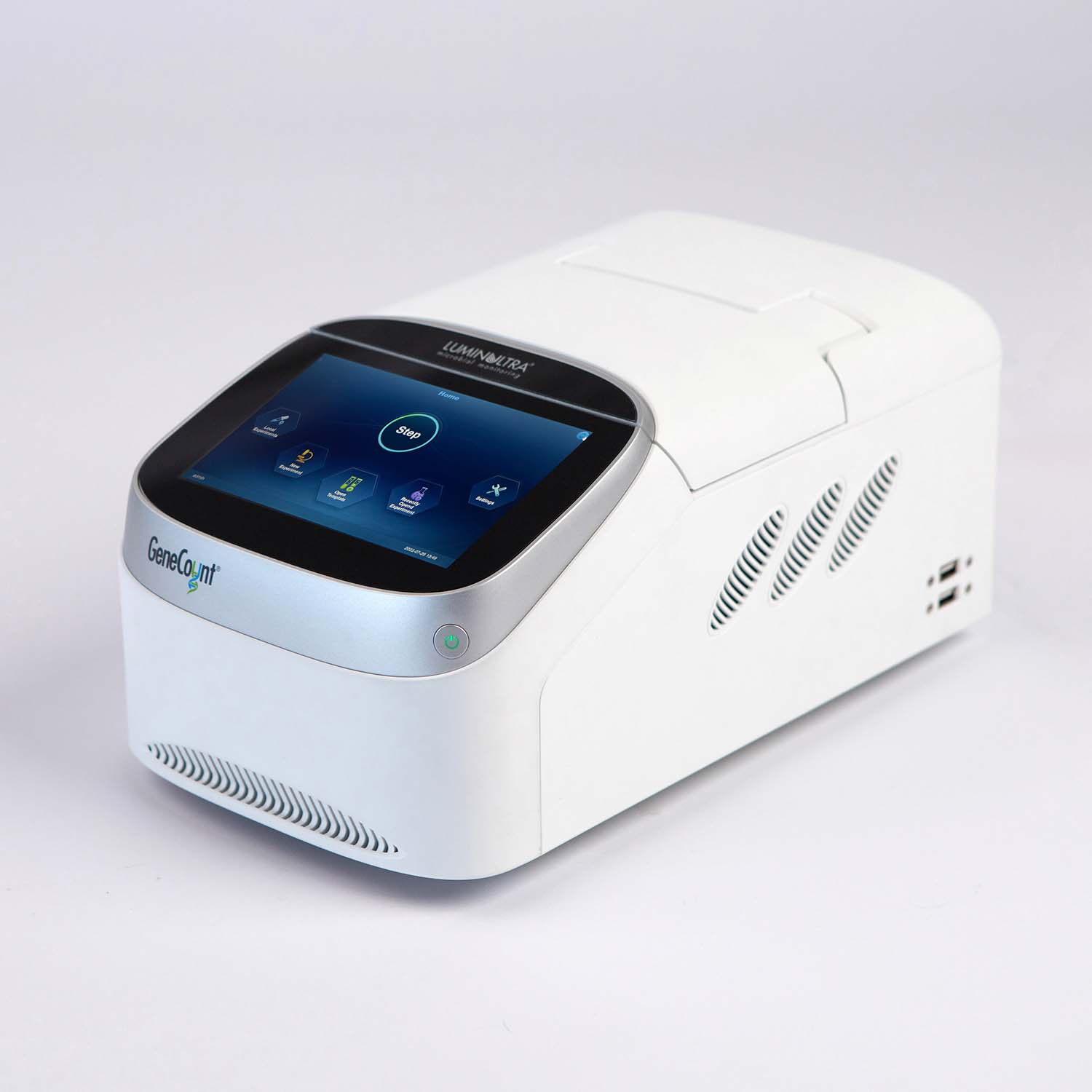
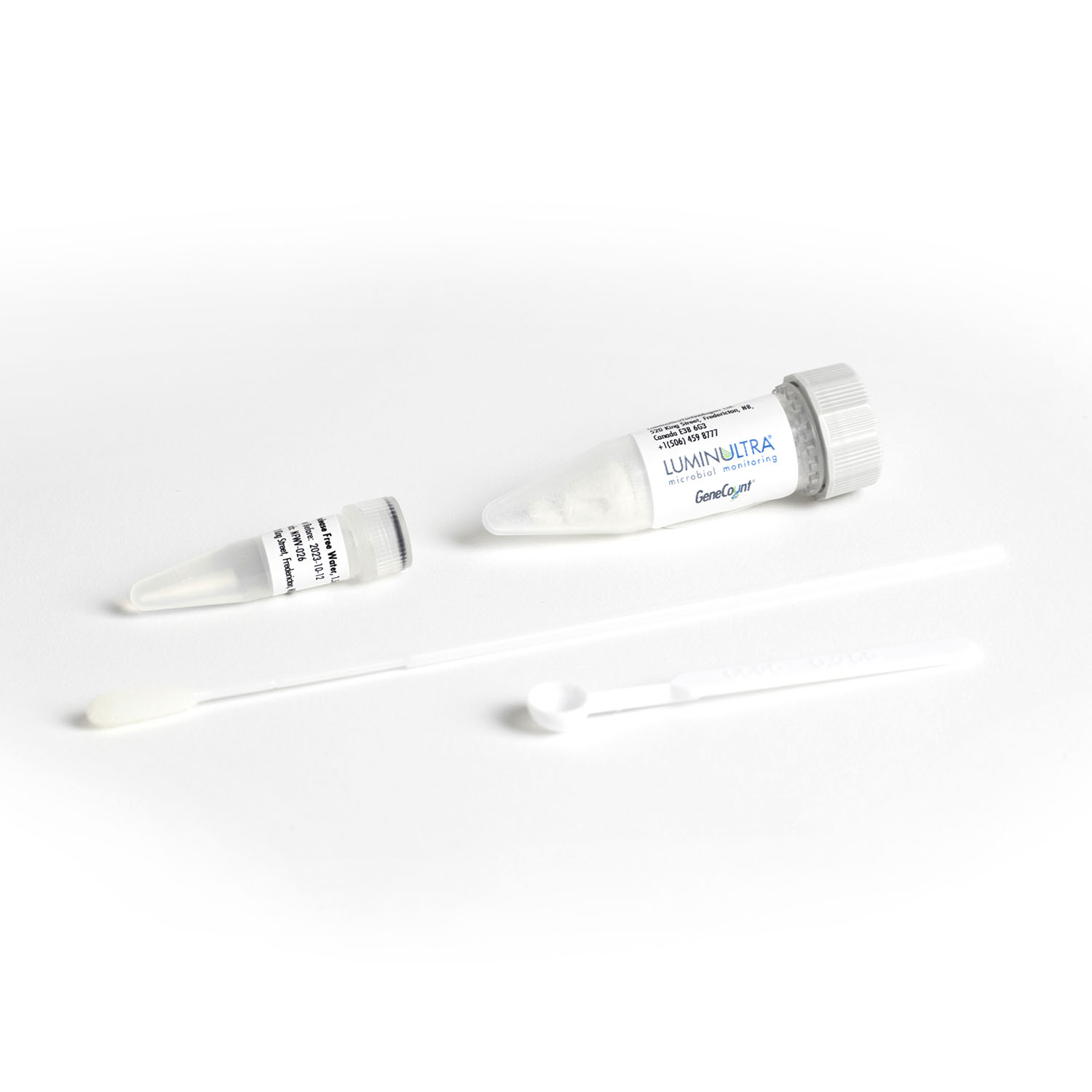
Accurate results in less than 2 hours
Our GeneCount® qPCR family of molecular testing solutions provides a complete suite to quickly and accurately identify the presence of Legionella, the bacteria that can cause Legionnaire’s disease. With LuminUltra you’ll get an end-to-end solution, including sample preservation, extraction and simple to use assay kits, resulting in a powerful addition to your testing protocol. Enable your clients to realize the benefits of a fast turnaround including increased worker safety, minimized regulatory risk exposure and reduced remediation costs.
Make informed decisions without waiting for culture tests

Accurate
Proven 100% accuracy with blind samples prepared for proficiency testing in CDC ELITE Labs.

Reliable
qPCR is an approved alternative method per governing bodies around the globe

Actionable
Support your clients’ needs by getting them the insights they need with a fast turnaround
qPCR for results you can trust
A technology gold-standard, qPCR is being increasingly used by molecular laboratories around the world due to its ability to quickly and accurately identify the presence of Legionella. Be confident in your actions and respond more quickly with this method rather than waiting 7-14 days for a culture test result. And trust that you’re getting a reliable result:
Detect viable but non-culturable cells: Detects all intact cells, including those that are viable and infectious but not culturable
Unlock a complete and accurate profile: Identify Legionella pneumophila and all Legionella species simultaneously, giving you a complete risk profile
CDC ELITE validation whitepaper
LuminUltra’s method is compliant with ISO/TS 12869 and meets technical performance criteria under 3rd party validation testing. A third-party validation of the LuminUltra qPCR solution using the Centers for Disease Control and Prevention (CDC) Environmental Legionella Isolation Techniques Evaluation (ELITE) program proficiency testing (PT) samples found that LuminUltra’s qPCR solution is 100% accurate at identifying Legionella species and Legionella pneumophila from blind samples prepared for proficiency testing at CDC ELITE Labs.

A solution that can scale with you
Realize the value of molecular testing immediately. Our GeneCount® suite of products include all of the elements you need to run your test, including sample preservation, extraction and simple to use assay kits. Get the right customized combination to meet your needs today, with the potential to build as you go.
- Adaptive: The GeneCount® product range has options from small footprint devices running 16 samples to large-scale high-throughput solutions of up to 96 samples with available automation and a variety of available assay kits.
- Supportive: We make getting started simple, including complete kits that include all the elements you need to run your tests, thorough onboarding support and decision-support software for interpreting results. Realize the value of your investment without delay.
- Flexible: Ask us about our range of purchasing options, including device rental with assay subscription.