This article was originally written for Water Quality Products by LuminUltra Technologies.
The COVID-19 pandemic led to significant shutdowns and reductions in commercial and travel activities. As people began working from home, office buildings operated at substantially reduced capacity, meaning that building water systems operated at reduced flows or were even shut down. Similarly, many passenger jets were grounded and cruise ships paused operations.
An unintended effect of these shutdowns is that, if not properly managed, stagnant water systems in buildings and on ships could provide a hospitable environment for pathogens to proliferate, like Legionella.
What is Legionella?
Legionella is a genus of bacteria that is found widely in aquatic environments, particularly in constructed water systems with warm temperatures and stagnant sections. Legionella are responsible for causing Legionnaires’ disease, a severe and potentially fatal pneumonia, as well as Pontiac Fever, a less severe infection. Legionnaires’ disease was first identified following an outbreak that occurred at an American Legion convention in Philadelphia in 1976. An investigation by the U.S. Centers for Disease Control and Prevention (CDC) identified Legionella pneumophila as the cause of the outbreak, which was estimated to have resulted in more than 200 infections and 29 deaths.
Symptoms of Legionnaires’ disease include: coughing, shortness of breath, fever, muscle aches, headaches, diarrhea, nausea and confusion. According to the CDC, healthy individuals are generally able to recover from infections, but often requires hospitalization. Individuals at increased risk from exposure include adults over the age of 50, smokers, heavy drinkers, or those with chronic lung disease, cancer, underlying illnesses or a weakened immune system. Complications from Legionnaires’ disease can result in lung failure or death.
Pontiac Fever is a less serious illness that can cause fever and muscle aches and the infection is typically cleared within a few days without medical treatment.
More than 50 Legionella species have been identified and these can be further divided by approximately 70 serogroups. In general, the majority of human infections are found to be caused by Legionella pneumophila serogroup 1, although approximately half of all Legionella species have been associated with Legionnaires’ disease.
Cases of Legionnaires’ disease have increased over the last 20 years, making Legionella one of the most significant sources of waterborne diseases in the U.S.
Learn why qPCR testing is a game changer for Legionella testing, and what the validation data has to say about its efficacy, benefits, and how it compares to other methods.
Why does testing matter for cruise ships?
Legionnaires’ disease can occur when a person inhales small water droplets containing Legionella bacteria. Common sources include cooling towers, showers and faucets, decorative fountains, and hot tubs. Cases have been linked to a variety of facility types, including hospitals, hotels, long-term care facilities and cruise ships. With 125 new cruise ships set to enter the world’s fleet by 2027, boosting the number of ships to more than 550 and providing capacity for about 40 million guests per year, maintaining passenger health is of great importance.
The most common sources of onboard waterborne illnesses, such as Legionnaires’, are from cooling water systems, faucets, showers, whirlpools, hot tubs and inline filters. Water heater temperatures under 50°C may increase the risk of Legionnaires’ disease due to bacterial growth in the tank, and the risk is particularly significant between 40 and 50°C.
While larger, newer passenger ferries and cruise ships have been built with more advanced sanitation solutions and adopted preventive measures — like encouraging passengers to sanitize hands upon entry to public spaces — the close proximity of thousands of passengers is bound to pose a public health challenge. Little has been done to protect passengers aboard smaller, older ships which, in many cases, have older water systems that are not as well maintained.
This was evident from the research paper Legionella Risk Assessment in Cruise Ships and Ferries, published in June 2017 by the US National Institutes of Health’s National Library of Medicine. Researchers evaluated the frequency and severity of Legionella contamination on 10 ferries and six cruise ships alongside or in transiting the Port of Messina, Sicily. From the water and air samples tested for qualitative and quantitative identification of Legionella, the researchers found that Legionella pneumophila serogroup 1, which is most frequently associated with cases of Legionnaires’ disease, was present in samples of the shower and tap water in 70% of the 10 ferries examined, and in 33% of the six cruise ships examined. They found Legionella pneumophila serogroups 2-14 in samples from 80% of the ferries and in samples from 16% of the cruise ships that were examined.
While Legionella contamination was not found in whirlpool bath, air or ice samples, they concluded that higher levels of Legionella contamination were found in local ferries and cruise ships, highlighting the need for corrective actions that are directly targeted for smaller vessels.
How to test for Legionella
A common method for environmental monitoring of Legionella is to use culture tests, which are typically performed in specialized laboratories following standard methods, such as those published by CDC or ISO. In general, water samples collected in the field are shipped on ice to a lab. The samples may then be filtered to concentrate the microbial cells, which are then suspended in a small volume of sterile water, or processed directly without filtration, depending on the water type and anticipated concentrations. The suspension is then added to a plate containing buffered charcoal yeast extract (BCYE) agar and incubated.
The culture technique has a relatively long history and many operators are familiar with the test. Various regulations and guidance documents are available, providing useful information to help operators assess their results. A significant disadvantage is that the test is slow, requiring several days to process and then for the lab to provide a report. Common turn-around times are in the range of 10 to 14 days, meaning it is not possible to use the data for routine operational changes.
The test may also underestimate the total amount of Legionella that are present. First, if cells are stressed, whether through exposure to biocide or through lack of nutrients, they may enter a viable but not culturable (VBNC) state. This means that the cells are alive, potentially still capable of causing infection, but will not grow by culture method. Additionally, the BCYE agar favours the growth of Legionella pneumophila over other species, which may also cause infections. Finally, Legionella often grow within protozoan hosts. These hosts provide a hospitable environment, sheltering Legionella from biocides, while also providing nutrients. Culture tests may often fail to detect Legionella that are growing within these hosts.
What is qPCR?
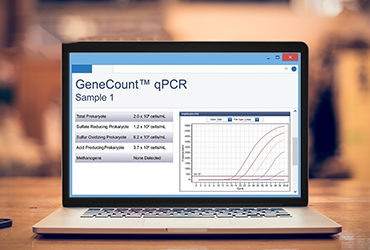
Another method for measuring Legionella is the use of qPCR. This is a rapid method that uses DNA to test for the target microorganism. One advantage of the qPCR method is that it is highly specific. The tests are designed to specifically detect a target genomic sequence that is unique to your target organism (e.g. Legionella pneumophila) or group of organisms (e.g. all Legionella species). More importantly, the method is rapid. The entire test, from sample collection to collecting the final measurement can be performed within hours, meaning results can be used to support operational decisions quickly.
While PCR techniques have been used widely for decades, like in clinical diagnostic applications, where qPCR is considered the gold-standard test method, its use in industrial and environmental applications has grown. This slower adoption can be partly explained by the fact that historically, qPCR was a lab-based technique, so its advantage as a rapid method was diminished by the fact that samples still needed to be shipped to a lab and then results may not be provided for several days. More recently, field-based kits have offered a unique solution to this, meaning that tests could be run in the field by operators, providing actionable results that could be obtained within two hours of sample collection. Moreover, qPCR tests are highly sensitive, making them suitable for performing screening tests.
One disadvantage of the qPCR-based approach is that it can potentially detect DNA from dead cells; however, these methods include filtration steps so DNA from lysed cells will pass through the filter and not be detected, meaning only DNA from intact cells can be detected. It is also worth noting that qPCR is recommended for routine monitoring, where the objective is to establish typical baselines and monitor for growth. Through this approach, any impact of dead cells is irrelevant, as dead cells do not grow.
Cruise ship water management
Given the complexity of on-board water systems and the potential for stagnation due to extended periods of down-time or holding time in system tanks, it is important for cruise ship operators to implement a water management program that includes effective monitoring and control operations.
A key component of the management plan is to include routine monitoring to frequently sample and monitor the water systems. qPCR testing is an ideal method for monitoring Legionella on cruise ships, where access to specialized laboratories is not available. If the data indicates that Legionella bacteria are proliferating then immediate action can be taken by system operators. This should include a risk analysis to find the root cause of the proliferation.
One very effective way to identify the source of proliferation is through microbial mapping of the network. This is achieved by sampling and analyzing the water in different locations of the system to identify the area of main proliferation. Once the source of contamination is identified, possible corrective actions could include adjustment of water temperatures for thermal disinfection or the flushing of system pipework to provide fresh water with biocide residual.
Reducing the risk
It is important to have an effective on-board water management program to ensure the safety and comfort of all guests and crew. To help reduce the risk of Legionella, this should include proactive monitoring.
Field qPCR provides an effective way to perform an overall screening program to identify potential hot-spots and then perform routine follow-up testing to monitor for growth, giving operators the opportunity to respond quickly with preventive actions.